The *NEW* Good Morning Thread!
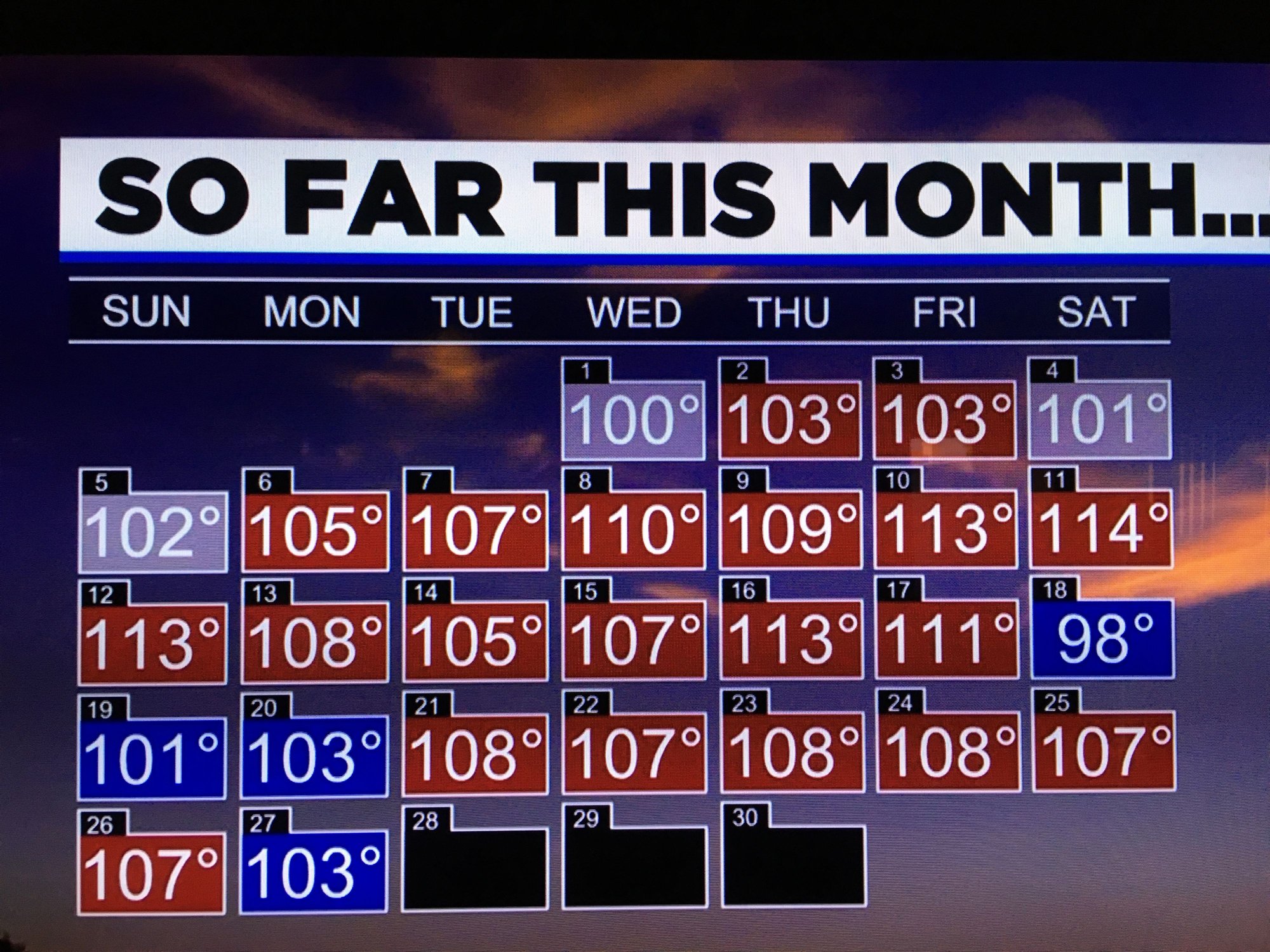
Yeah I saw the national weather map and y’all have a frontal system with a lotta rain. That same system grazed us, which resulted in an infusion of moist air and the rain we got (0.1” total). We have increased humidity and thunderstorm chances all week long. And highs in the low 100s. I’ll take this over 110+ any day.
Sunrise is at 5 am.
I am not up that early, and by the time I am up, the sun has been broiling the landscape for a couple of hours and it’s hide from the sun time.
Very nice pics Dan, I hope you are there when I do the Model t thing. Had a great time with my bro and sis at Tawas State park. Wifey and I stayed a night with the campers. The park is on a small peninsula with a old light house on tip of it. Lake Huron on the east and the Tawas bay on the west. So always a little cooler than surrounding area from cool breeze off water. Good times!!
Very nice pics Dan, I hope you are there when I do the Model t thing. Had a great time with my bro and sis at Tawas State park. Wifey and I stayed a night with the campers. The park is on a small peninsula with a old light house on tip of it. Lake Huron on the east and the Tawas bay on the west. So always a little cooler than surrounding area from cool breeze off water. Good times!!
Good morning guys,
Well, since I was posting yesterday afternoon, obviously the Car Show didn't happen for me. It was still raining at my place at 1100, and registration closed at 1200, so the roads were gonna be wet. I don't know if they had it or not, the flyer didn't list a rain date?
Got out for about a 2-hour ride early yesterday evening. The roads were dry by that time, and it was nice and cool behind the front. I'm gonna ride with my buddy who own's the '66 Vette on Wednesday. I have planned a 280-mile roundtrip ride southwest of me to a place called Echo Bluff State Park, riding exclusively back country 2 lane roads. I have proposed it to my buddy and I'm waiting to hear back if he's willing to ride that far/long. It would be about a 7 & 1/2-hour day including an hour and a half for lunch/gas. This distance is on the far end of what he's usually willing to tolerate, but in his defense, his bike is not as large and comfy as mine.
No big plans for this week. We're not looking forward to the weekend of the 4th and the local fireworks that will be set off by neighbors, and others close by, because they torment Monty so badly. There are 3 car shows over the weekend of the 4th, on the 2nd, 3rd, and 4th. I'd like to make it to 2 of the 3. I went to the one on the 2nd last year and it was nice. Right now the forecast is not great for any of the 3-days, but it's really too damn early to tell.
Hope everyone has a wonderful day!
Well, since I was posting yesterday afternoon, obviously the Car Show didn't happen for me. It was still raining at my place at 1100, and registration closed at 1200, so the roads were gonna be wet. I don't know if they had it or not, the flyer didn't list a rain date?
Got out for about a 2-hour ride early yesterday evening. The roads were dry by that time, and it was nice and cool behind the front. I'm gonna ride with my buddy who own's the '66 Vette on Wednesday. I have planned a 280-mile roundtrip ride southwest of me to a place called Echo Bluff State Park, riding exclusively back country 2 lane roads. I have proposed it to my buddy and I'm waiting to hear back if he's willing to ride that far/long. It would be about a 7 & 1/2-hour day including an hour and a half for lunch/gas. This distance is on the far end of what he's usually willing to tolerate, but in his defense, his bike is not as large and comfy as mine.
No big plans for this week. We're not looking forward to the weekend of the 4th and the local fireworks that will be set off by neighbors, and others close by, because they torment Monty so badly. There are 3 car shows over the weekend of the 4th, on the 2nd, 3rd, and 4th. I'd like to make it to 2 of the 3. I went to the one on the 2nd last year and it was nice. Right now the forecast is not great for any of the 3-days, but it's really too damn early to tell.
Hope everyone has a wonderful day!
Hi everyone.
Gosh this no internet/troublesome internet thing at home doesn't seem to bother me like I thought it would. I must have been spending too much time looking at it. Facebook? What's that. I can look at my car forums at work. Maybe my bad skin will clear up now.
So it was back to work this morning. We've had a good day, got done early. Lots of wheels being built for a head start tomorrow. This isn't the work I left a week ago, I wonder what happened.
So I was supposed to have my new AC installed on Thursday, but I can't get the day off work. Not sure what to do. Can't call in sick because I already asked about it. I guess Tiger will have to stay at Mom's a while longer. Actually he's going to jail over the weekend, since I'm going to Florida and I don't want him to freak out about fireworks when he's with Mom. He normally doesn't but just in case.
So we were supposed to have some yard people come and do a big cleanup of the yard at the lake, and I called Mom to see how they were doing. It seems, they didn't remember they were going to be here today and instead will be here tomorrow and Wednesday. Hm. I want to call the whole thing off but Mom says no I'm not capable of doing it myself. Just because I haven't doesn't mean I can't. I'd just add it to the list of things to do. Eh, it's her money I can't tell her how to spend it.
Okay gonna clean up and hit the road. Not so hot out today, it's supposed to rain most of the week but I'll believe it when I see it. Maybe I can have only one fan going tonight instead of two. Have a good day everyone.
Gosh this no internet/troublesome internet thing at home doesn't seem to bother me like I thought it would. I must have been spending too much time looking at it. Facebook? What's that. I can look at my car forums at work. Maybe my bad skin will clear up now.
So it was back to work this morning. We've had a good day, got done early. Lots of wheels being built for a head start tomorrow. This isn't the work I left a week ago, I wonder what happened.
So I was supposed to have my new AC installed on Thursday, but I can't get the day off work. Not sure what to do. Can't call in sick because I already asked about it. I guess Tiger will have to stay at Mom's a while longer. Actually he's going to jail over the weekend, since I'm going to Florida and I don't want him to freak out about fireworks when he's with Mom. He normally doesn't but just in case.
So we were supposed to have some yard people come and do a big cleanup of the yard at the lake, and I called Mom to see how they were doing. It seems, they didn't remember they were going to be here today and instead will be here tomorrow and Wednesday. Hm. I want to call the whole thing off but Mom says no I'm not capable of doing it myself. Just because I haven't doesn't mean I can't. I'd just add it to the list of things to do. Eh, it's her money I can't tell her how to spend it.
Okay gonna clean up and hit the road. Not so hot out today, it's supposed to rain most of the week but I'll believe it when I see it. Maybe I can have only one fan going tonight instead of two. Have a good day everyone.
I ran an Appleseed this weekend and it was horribly hot Saturday and warm and muggy Sunday. It was rough the first day.
Now I am back at work after my week off. I got about 66% of my stuff done I wanted to do.
Now I am back at work after my week off. I got about 66% of my stuff done I wanted to do.
John - I bet it was tough on Saturday, I know how hot it was here. Sure no fun being on the range when it's that hot. You got 2/3 finished of what you had planned to get done, that's better than my usual %.
Question for the learned
I'm going to clean the curd out of my jeep tank, 1st vacuum out in particles I can, ,scrub with wire brush, repeat vacuum
I bought sum "Evaporust." That I'm gonna put in the jeep tank for a week. Then pump it out with a small electric pump/ What would you use after you got the majority of it out to clean any residue out. Water? Kerosine? Diesel fuel? gasoline?
I'm going to clean the curd out of my jeep tank, 1st vacuum out in particles I can, ,scrub with wire brush, repeat vacuum
I bought sum "Evaporust." That I'm gonna put in the jeep tank for a week. Then pump it out with a small electric pump/ What would you use after you got the majority of it out to clean any residue out. Water? Kerosine? Diesel fuel? gasoline?
Jim - I looked at some Evaporust stuff on the web. I also found the Proprietary (active) ingredient. The active ingredient is tannic acid. Tannic acid is highly soluble in both alcohol & water. IMO, if you have some on the farm, I'd pour a gallon of denatured methanol or dentatured ethanol in the tank after you removed the Evaoprust. Then remove the alcohol. Then wash out with water & remove the water. I wouldn't use diesel or kerosene or any petroleum product until you remove the Evaporust with alcohol and then water.
EDIT: Actually, if you could fill the tank w/ alcohol that would be best (but expensive @ $35/gal.). Leaving a gallon of 190 proof denatured alcohol in the tank for an extended period of time should work well.
EDIT the EDIT: You might consider a gallon of alcohol and fill the tank 1/2 full by adding water so as to dilute the alcohol might be a possibility, as well. The more alcohol you have in the tank the better the removal of the tannic acid.
EDIT: Actually, if you could fill the tank w/ alcohol that would be best (but expensive @ $35/gal.). Leaving a gallon of 190 proof denatured alcohol in the tank for an extended period of time should work well.
EDIT the EDIT: You might consider a gallon of alcohol and fill the tank 1/2 full by adding water so as to dilute the alcohol might be a possibility, as well. The more alcohol you have in the tank the better the removal of the tannic acid.
Last edited by Vintage Chief; June 27th, 2022 at 06:12 PM.
Norm, I am trying to clean it w/o removing it. I have found out , all of the places that clean them out have been closed down by the EPA So, I'm trying to do a good clean w/o acid
How long do I need to leave the alcohol in the tank?
How flammable is a 50/50 mix of alcohol and water?
I'm a little concerned about 'Flash Rust" returning before the tank is dry. What do you think of spraying WD-40 in the tank to stop the possibility of Flash Rust
Tanks for all of your help
Good morning everyone.
Not a bad night, it cooled off after some rain and I only needed one fan. I sure hate being home without Tiger.
Jamesbo is the Jeep sucking up trash from the tank and running poorly, or is this just something you want to do? Fill it up with some premium and dump in a quart of ATF, then drive it down that path to the lake you took me on in the Gator. That will clean it out.
The overtime people signed 61 wheels this morning. That sounds great on the surface, but it will just make it easier for the powers that be to up the number. Doesn't matter to me what the number is but the people that actually do the work are always saying the number is too high. This is not the way to combat it.
We'll see if the yard people show up today. I kind of hope they don't. Something just doesn't sit right with me about them.
Okay break time. Don't want to miss that. Have a good day everyone.
Not a bad night, it cooled off after some rain and I only needed one fan. I sure hate being home without Tiger.
Jamesbo is the Jeep sucking up trash from the tank and running poorly, or is this just something you want to do? Fill it up with some premium and dump in a quart of ATF, then drive it down that path to the lake you took me on in the Gator. That will clean it out.
The overtime people signed 61 wheels this morning. That sounds great on the surface, but it will just make it easier for the powers that be to up the number. Doesn't matter to me what the number is but the people that actually do the work are always saying the number is too high. This is not the way to combat it.
We'll see if the yard people show up today. I kind of hope they don't. Something just doesn't sit right with me about them.
Okay break time. Don't want to miss that. Have a good day everyone.
Jim - Petroleum gasoline is twice as volatile as ethyl alcohol (ETOH = Ethanol).
If you use tannic acid (Evaporust) there would be no formation of surface rust as tannic acid coats the iron-based metal [much the same way phosphate coating (Phosphoric acid) protects iron-based metal to prevent rust because it renders the remaining iron with a coating of Iron Phosphate (FePO4)].
If you're not going to use Evaporust (tannic acid) then you have no need to use alcohol since you said you don't want to use an acid (I'm confused).
Yes, spraying the tank w/ WD-40 would/could/might prevent surface rust. Honestly, the amount of (if any) surface rust rendered in say one week's time is the least of your issues if you really, really have a serious rust problem. Why not invest in a borescope/endoscope and really look inside the tank to see just how much rust & gunk is in there to start with?
If you use tannic acid (Evaporust) there would be no formation of surface rust as tannic acid coats the iron-based metal [much the same way phosphate coating (Phosphoric acid) protects iron-based metal to prevent rust because it renders the remaining iron with a coating of Iron Phosphate (FePO4)].
If you're not going to use Evaporust (tannic acid) then you have no need to use alcohol since you said you don't want to use an acid (I'm confused).
Yes, spraying the tank w/ WD-40 would/could/might prevent surface rust. Honestly, the amount of (if any) surface rust rendered in say one week's time is the least of your issues if you really, really have a serious rust problem. Why not invest in a borescope/endoscope and really look inside the tank to see just how much rust & gunk is in there to start with?
I haven't been on this thread for quite a while but still follow you guys. I have attached a link to a gas tank kit by Bill Hirsch. It would require you to remove the tank. I used the smaller version on a 46 Cushman scooter I have and it worked great. I kept cleaning the tank our but could never get it completely clean. I little crude would still break loose and make it's way to the carb. The sealer leaves a nice white coating in the gas tank. Here is the link.
Bill Hirsch Automotive Gas Tank Repair Kit - TP Tools & Equipment
Bill Hirsch Automotive Gas Tank Repair Kit - TP Tools & Equipment
Well - I can't read your mind, Jim. Was I supposed to know you meant muriatic acid (HCl = hydrochloric acid)? Maybe you meant sulfuric acid, phosphoric acid, acetic acid, formic acid, nitric acid, citric acid (to name only several). I clearly stated Evaporust is made of tannic acid. Now, do you see how confusing this is when I state Evaporust is made of tannic acid and you state you don't want to use an acid? I'm sorry, I can't read your mind.
BTW, there is nothing mysterious and miraculous about Bill Hirsch products. The Gas Tank Etch solution is nothing more than 45% phosphoric acid dissolved in (1%-5%) 2-propanol & (1%-5%) methanol (both alcohols) - absolutely not a Miracle of any figment of the imagination. To be extremely clear about this - you absolutely cannot with any certainty determine what is and is not going to work unless you look INTO the tank to visualize the metal & rust. All bets are off unless you look inside the tank.
All I said is the damn stuff worked good on the gas tank I did. I don't give a **** what is in it as long as it works. Now I remember why I quit participating on this thread and you will never see me make a post on here again.
Dan - No offense to you or Kenneth, but I think I'll stay just where I'm at! Long summers, but not as brutally hot as what Kenneth has, and short, mild winters, with very little snow. For someone whose major hobbies revolve around motorsports, our weather's pretty darn nice. The biggest downside is being in Tornado Alley, it can be a little scary at times.
I'm not a mind reader either. I "thought" the instructions [above] you gave me were pretty clear.
That's what I'm gonna do w/o looking in the tank to see the rust that I know is in there because, it's in the fuel filter
BTW 'IF" [which I'm not going to do] I bought an endoscope [which I'm not going to do] and looked into the tank, I'm not real sure I am capable of determining anything different about different kinds of rust
IMHO rust is rust
But tanks for your help anywho
Jim - My own personal approach would be this - the only caveat I have to my approach is this. I would want to look into the tank to determine the amount of rust and the current integrity of the remaining metal.
My approach:
(1) I would use a 50:50 solution of (20%) phosphoric acid. This would yield a 10% solution of phosphoric acid. By far the best acid to remove rust and etch the metal. The metal which remains would then be likened to providing a phosphate layer of FePO4 onto the remaining good metal. This process (employing a chemical technique) is absolutely unlike an electrical phosphating technique. Instead of imparting an electrical field to the metal(s), this method employs only chemical electrolysis. This will render the metal with a corrosion resistant FePO4 (iron phosphate)
protective layer. Leave the phosphoric acid solution for ~48 hours to ~96 hours. Again, you have absolutely no Earthly notion of what the inside of your tank looks like - therefore, I have to be very honest - it's a guess.
(2) I would then remove the phosphoric acid.
(3) I would then fill the tank with an approximate 50:50 mixture of ethanol & allow to remain 24 hours.
(4) Drain the ethanol.
(5) Two very thorough rinses of 100% H20.
There is absolutely no need to neutralize phosphoric acid with a base. Phosphoric acid is not a strong acid and as I previously stated, cleaning w/ phosphoric acid will yield a corrosion resistant FePO4 (Iron phosphate) layer. This is nearly the same a phosphating metal.
The primary delta here is which oxide of iron you are removing. There are two forms of rust - Fe2+ (ferrous) & Fe3+ (ferric) iron oxides are both rusts. Phosphoric acid removes ferric (Fe3+) iron (rust). Oxalic acid removes ferrous (Fe2+) iron (rust) primarily. In both chemical processes we're rendering these cations (Fe2+ & Fe3+) soluble in solution. The solution we're using is going to be an acid - either phosphoric acid or oxalic acid. When you look at the phosphoric acid solution &/or the oxalic acid solution when this process is complete, you'll note the solution has turned nearly a complete black color (maybe somewhere around very, very deep green to black). That's the cations of Fe2+ & Fe3+ dissolving into the solution (phosphoric acid or oxalic acid). NOTE: If you elect to use Evaporust, there is nothing wrong with using Evaporust. The tannic acid in Evaporust is quite similar to phosphoric acid, but it is also quite dissimilar to phosphoric acid. Tannic acid is an organic acid while phosphoric acid is an inorganic acid. Because tannic acid is an organic acid, it contains carbon (C) and rightfully so as compared to an equivalent inorganic counterpart, tannic acid is a weaker acid than phosphoric acid. None-the-less, tannic acid will take longer, but it will also to a very admirable job of removing rust - but it's going to be slower than phosphoric acid. BTW, if using tannic acid instead of phosphoric acid, the resulting corrosion protecting is caused by the metal layer having an iron oxalate layer rather than an iron phosphate layer.
Again, I personally would use phosphoric acid as opposed to tannic acid (contained in Evaporust). But that's your choice. Industrial processes employ both tannic acid and phosphoric acid to protect iron metals from rusting. In the above description, you can substitute Evaporust (tannic acid) for my usage of phosphoric acid in the same concentrations.
My approach:
(1) I would use a 50:50 solution of (20%) phosphoric acid. This would yield a 10% solution of phosphoric acid. By far the best acid to remove rust and etch the metal. The metal which remains would then be likened to providing a phosphate layer of FePO4 onto the remaining good metal. This process (employing a chemical technique) is absolutely unlike an electrical phosphating technique. Instead of imparting an electrical field to the metal(s), this method employs only chemical electrolysis. This will render the metal with a corrosion resistant FePO4 (iron phosphate)
protective layer. Leave the phosphoric acid solution for ~48 hours to ~96 hours. Again, you have absolutely no Earthly notion of what the inside of your tank looks like - therefore, I have to be very honest - it's a guess.
(2) I would then remove the phosphoric acid.
(3) I would then fill the tank with an approximate 50:50 mixture of ethanol & allow to remain 24 hours.
(4) Drain the ethanol.
(5) Two very thorough rinses of 100% H20.
There is absolutely no need to neutralize phosphoric acid with a base. Phosphoric acid is not a strong acid and as I previously stated, cleaning w/ phosphoric acid will yield a corrosion resistant FePO4 (Iron phosphate) layer. This is nearly the same a phosphating metal.
The primary delta here is which oxide of iron you are removing. There are two forms of rust - Fe2+ (ferrous) & Fe3+ (ferric) iron oxides are both rusts. Phosphoric acid removes ferric (Fe3+) iron (rust). Oxalic acid removes ferrous (Fe2+) iron (rust) primarily. In both chemical processes we're rendering these cations (Fe2+ & Fe3+) soluble in solution. The solution we're using is going to be an acid - either phosphoric acid or oxalic acid. When you look at the phosphoric acid solution &/or the oxalic acid solution when this process is complete, you'll note the solution has turned nearly a complete black color (maybe somewhere around very, very deep green to black). That's the cations of Fe2+ & Fe3+ dissolving into the solution (phosphoric acid or oxalic acid). NOTE: If you elect to use Evaporust, there is nothing wrong with using Evaporust. The tannic acid in Evaporust is quite similar to phosphoric acid, but it is also quite dissimilar to phosphoric acid. Tannic acid is an organic acid while phosphoric acid is an inorganic acid. Because tannic acid is an organic acid, it contains carbon (C) and rightfully so as compared to an equivalent inorganic counterpart, tannic acid is a weaker acid than phosphoric acid. None-the-less, tannic acid will take longer, but it will also to a very admirable job of removing rust - but it's going to be slower than phosphoric acid. BTW, if using tannic acid instead of phosphoric acid, the resulting corrosion protecting is caused by the metal layer having an iron oxalate layer rather than an iron phosphate layer.
Again, I personally would use phosphoric acid as opposed to tannic acid (contained in Evaporust). But that's your choice. Industrial processes employ both tannic acid and phosphoric acid to protect iron metals from rusting. In the above description, you can substitute Evaporust (tannic acid) for my usage of phosphoric acid in the same concentrations.
I'm not a mind reader either. I "thought" the instructions [above] you gave me were pretty clear.
That's what I'm gonna do w/o looking in the tank to see the rust that I know is in there because, it's in the fuel filter
BTW 'IF" [which I'm not going to do] I bought an endoscope [which I'm not going to do] and looked into the tank, I'm not real sure I am capable of determining anything different about different kinds of rust
IMHO rust is rust
But tanks for your help anywho
That's what I'm gonna do w/o looking in the tank to see the rust that I know is in there because, it's in the fuel filter
BTW 'IF" [which I'm not going to do] I bought an endoscope [which I'm not going to do] and looked into the tank, I'm not real sure I am capable of determining anything different about different kinds of rust
IMHO rust is rust
But tanks for your help anywho
Put another way. Metal iron is derived from metal ore. Iron ore exists in its most natural state - e.g. as an ore - it is highly STABLE. When we extract iron (Fe) we process the iron ore. We remove contaminants and heat it to ~2500°F until it is molten so we can form iron (Fe) into molds and employ the iron for various usages. At this point (your common everyday iron used to make engines, piping, etc. ad infinitum) is completely unlike any iron ore which we harvested from the soil - it is now in its most UNSTABLE form. It is completely unlike iron ore. It is now completely at the mercy of oxygen & water which oxidizes the iron (turns it into rust) - either Fe2+ or Fe3+. It's simple Redox reactions governed by simple Redox equations. The iron is oxidized (Fe2+ & Fe3+) into rust in the presence of oxygen & water. You want to remove it? Then you use the process of reduction - e.g. a Redox reaction to make the Fe2+ and/or Fe3+ soluble in a solute (solution). Oxidation & reduction. When something is oxidized it loses electrons, when something is reduced it gains electrons. What we're doing is reducing the iron into a soluble state in order to remove the rust.
Last edited by Vintage Chief; June 28th, 2022 at 03:16 PM.
Tanks, Norm.
I still plan on going ahead, IMHO if it's beyond repair, I would think it would leak. If it leaks, I'll yank it out and see if it can be patched or tossed
wish me luck welding a fuel tank IF i have to
I still plan on going ahead, IMHO if it's beyond repair, I would think it would leak. If it leaks, I'll yank it out and see if it can be patched or tossed
wish me luck welding a fuel tank IF i have to